SINGAPORE, 2021-Jul-7 — /EPR BIOTECH NEWS/ — Austrianova (the SG Austria group, Austrianova Singapore and Austrianova Thailand) is pleased and honoured to announce that, after extensive due diligence, Real Tech Fund (managed by Real Tech Holdings, Japan) has invested an undisclosed seven figure amount in Austrianova via its Real Tech Global Fund 1 to accelerate Austrianova’s growth. Real Tech Fund is a venture capital firm that invests specifically in innovative deep-technology startups mainly in Japan and Southeast Asia. Austrianova was assisted in this transaction by Square Associates. The financing from Real Tech coincides with, as well as bolsters, the Company’s next stage of growth in production capacity in order to satisfy the strong demand witnessed across the wide variety of applications that its unique protective cell encapsulation technology addresses. It also enhances and facilitates Austrianova’s entry into the Japanese market and specifically partnerships with clients benefiting from Austrianova’s Cell-ina-Box® and Bac-in-a-Box® technologies.
“We are pleased that Real Tech has decided to invest in Austrianova as well as that they are convinced of the value of the Cell-in-a-Box® and Bac-in-a-Box® technologies for solving serious problems faced by the planet and by humanity” stated Walter H Gunzburg, Chairman of Austrianova. “Real Tech already has proven to be a valued partner as well as a significant investor in Austrianova and we look forward to working closely together in the future†added Brian Salmons, CEO of Austrianova.

About Austrianova
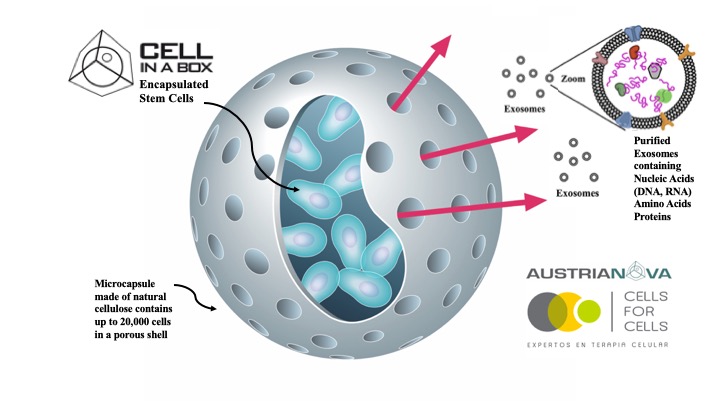
Austrianova (the SG Austria Group), is a biotech company with a global footprint and operations in Singapore and Thailand. Austrianova utilizes a novel and proprietary technology for the encapsulation of living mammalian (Cell-in-a-Box®) and bacterial (Bac-in-a-Box®) cells. Cell-in-a-Box® protects the encapsulated cells from rejection by the immune system, allows cells to be easily transported, stored and implanted at specific sites in patients. The
technology, which has been proven safe and efficacious in clinical trials carried out in Europe, allows companies to develop any kind of cells as a one-for-all living pharmaceutical. Bac-in-a-Box® is a similar protective device adapted for encapsulation of probiotic bacteria where it has human food and animal feed applications as well as rebalancing the microbiome due to its ability to extend storage under lyophilized conditions and to protect encapsulated bacteria against destruction by stomach acid. Austrianova now also offers GMP4Cells that includes competitively priced Master Cell Bank and Working Cell Bank production as well as “Fill and Finish†services for cell therapy products (such as stem cell therapies, biologics produced from cells e.g. vaccines, antibodies, enzymes, recombinant proteins, exosomes etc).
About Real Tech
Real Tech Holdings / Real Tech Fund is a joint venture fund between euglena Co.,Ltd and Leave a Nest Co., Ltd. It supports change makers who give their heart and soul to solve societal and environmental challenges. Real Tech Holding’s manages Real Tech Fund, Japan’s leading deep-tech focused venture capital fund.
For more information: https://www.realtech.holdings
Forward-Looking Statements
This release includes forward-looking statements regarding Austrianova (the Company) and its respective businesses. Such statements are based on the current expectations of the management of each entity. The forward-looking events and circumstances discussed in this release, including completion of the public offering, may not occur and could differ materially as a result of known and unknown risk factors and uncertainties affecting the Company, including risks affecting the Company, economic factors and the equity markets generally. No forward-looking statement can be guaranteed. Except as required by applicable securities laws, forward-looking statements speak only as of the date on which they are made and Austrianova undertakes no obligation to publicly update or revise any forwardlooking statement, whether as a result of new information, future events, or otherwise. This is not an offer or solicitation to buy or sell any securities.
Financial Information Contact: pierre.faddoul@square-associates.com
Company and Technology Contact: salmons@sgaustria.com
For more information: http://www.austrianova.com
SOURCE Austrianova