BOLZANO/ BOZEN, Italy, 19-Mar-2023 — /EPR BIOTECH NEWS/ — XSpline SPA, a start-up company incorporated in Bolzano (Italy) and the Ordensklinikum Linz Elisabethinen Hospital in Linz (Austria) today announced the first patient enrolled in a clinical study (ClinicalTrials.govID: NCT05327062) for the cardiac resynchronization therapy (CRT) guided by non-invasive electrical and venous anatomy assessment.  The international multicenter prospective study will include 150 patients from 16 centers in the U.S. and Europe.
XSpline Cloud provides a cloud-based non-invasive cardiac panoramic mapping technology to select individual CRT treatment strategy and predict outcomes.
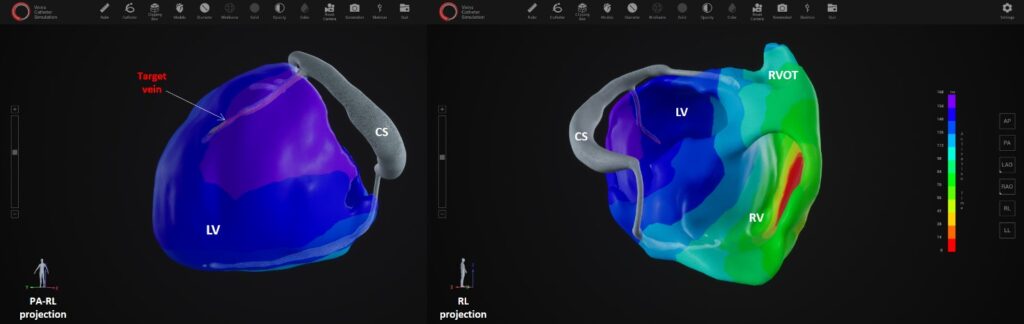
Fast and fully automatic Artificial Intelligence based epi- and endocardial segmentation of cardiac structures, including coronary sinus veins, provides clear understanding of individual patient’s anatomy. The electrical assessment is based on a 3D panoramic electro-anatomical map based on 12 -lead ECG only, without requiring multichannel ECG recording or any kind of body surface potential mapping. XSpline Cloud is the first system in the world with AI based identification of the correct target zone for LV lead implantation.
The cloud-platform guarantees full operability with existing clinical standards and data formats; it supports IHE profiles, HL7 FIHR, DICOM, and a broad range of ECG formats. The platform also provides embedded medical grade visualization tools, such as an integrated multimodality DICOM viewer, an ECG viewer with measurement capabilities, a high-performance 3D viewer for segmented cardiac structures and electro- anatomical maps as well as an interactive navigator for LV lead placement.
Dr. Georgios Kollias, M.D., is the principal investigator for the clinical trial in Austria and performed the first procedure on a 71-year-old patient with ESV LV of 154 ml and EF LV of 20%.
“The XSpline Cloud software provides a unique approach to selecting an individualized CRT treatment strategy. It is a comprehensive tool for successful CRT implantation that can also predict outcomes, making the implantation procedure faster, easier, and safer for the patient. We are very pleased to start using this tool in our clinicâ€, said Dr. Kollias.
“XSpline SPA is supported by an international R&D team of skilled clinicians, mathematicians, and biomedical engineers with the shared goal of improving patient care. We think that the real-time visualization of each patient’s individual anatomy, the precise non-invasive electro-anatomical endo- and epicardial activation map and the interactive navigator will help to increase CRT respondersâ€, said Mr. Werner Rainer, CEO of the company.
XSpline Cloud is an investigational device and not yet approved for commercial use.
SOURCE: EuropaWire